Following the successful completion of the CBSE Term 1 exam, the Board is preparing to conduct CBSE Term 2 exams. Students are advised to ride their practice horses onto the battlefield. The exam is likely to be held in March -April 2022 for the remaining 50% Syllabus. In this blog post, we are going to share the carbon and its compounds class 10 important questions and answers.
The Syllabus of CBSE class 10 term 2 exams covers 4 units namely:
| Unit | Term-II | Marks |
|---|---|---|
| I. | Chemical Substances-Nature and Behaviour (Chapter 4 & 5) | 10 |
| II. | World of Living (Chapter 8 & 9) | 13 |
| III. | Effects of Current (Chapter 12 & 13) | 12 |
| IV. | Natural Resources (Chapter 15) | 05 |
The first unit constitutes of two chapters i.e., Carbon and its compounds (chapter 4) and Periodic classification of elements (chapter 5). Here we are giving you the complete glance of carbon and its compounds class 10 important questions and answers and what to study from this chapter 4. The chapter focuses on the covalent properties and versatility of Carbon in forming a wide range of saturated and unsaturated compounds in the form of branches, chains, or rings.
To help you understand the chapter, a brief overview of alkanes, alkenes, alkynes, isomers, carbon allotropes, and the fundamentals of a homologous series are included in the chapter, you can find similar questions in CBSE Term 2 science question Bank by MTG.
Carbon and its Compounds Class 10 Important Questions and Answers
We’ve compiled a list of six important questions about carbon and its compounds that you should not miss while studying for the CBES term 2 exams.
Q.1 Case: Read the passage given below and answer the following questions from I to V.
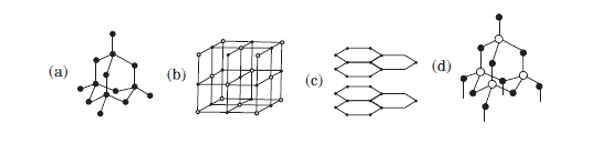
Two allotropic forms of carbon which are crystalline in nature, are diamond and graphite. They differ physically but chemically they are similar. Diamond is the hardest crystalline form of carbon. In diamonds, each carbon atom is linked to four other carbon atoms by covalent bonds. In graphite, each carbon atom is linked to three other carbon atoms by a covalent bond. Graphite is relatively soft and greasy. It is also a good conductor of electricity. The C — C bond length in graphite is 141.5 pm while in diamond it is 154 pm.
I.Which of the following is a good conductor of heat and electricity?
| (a) Coal | (b) Diamond | (c) Charcoal | (d) Graphite |
Ans. (d) Graphite
II.Graphite is a good conductor of electricity because
(a) it has free electrons
(b) it has free atoms
(c) it is crystalline
(d) it is soft and greasy.
Ans. (a) Graphite is a good conductor of electricity because it has free electrons.
III.Which of the following types of binding forces is present in the structure of diamond?
| (a) Ionic | (b) van der Waals’ | (c) Covalent | (d) None of these |
Ans. (c) Covalent
IV.Diamond is not a good conductor of electricity because
(a) it is very hard
(b) its structure is very compact
(c) it is not water-soluble
(d) it has no free electron.
Ans. (d): In diamond, one carbon is attached to four other carbon atoms hence it has no free electron.
V.Which of the following is the structure of a diamond?
You will also like – How to Solve the MCQ Based Questions
Q.2 Covalent compounds have low melting and boiling point. Why?
Ans. Covalent compounds have low melting and boiling points because the forces of attraction between molecules of covalent compounds are very weak.
Q.3 Name a cyclic unsaturated carbon compound?
| (a) Methane | (b) Ethyl Alcohol | (c) Benzene | (d) Graphite |
Ans. (c) Benzene is a cyclic unsaturated carbon compound.
Q.4 Assertion (A): Following are the members of a homologous series: CH3OH, CH3CH2OH, CH3CH2CH2OH
Reason (R): A series of compounds with the same functional group but differing by —CH2 unit is called homologous series.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Ans. (a): The given compounds are members of homologous series of alcohol.
Q.5. (a) What is a homologous series of compounds? List any two of its characteristics.
(b) What is the next higher homologue of C3H7OH? What is its formula and what is it called?
Ans. (a) A homologous series is the family of organic compounds having the same functional group, similar chemical properties but the successive (adjacent) members of the series are differ by a CH2 unit or 14 mass units. Two characteristics of homologous series are:
(i) The successive compounds of the homologous series differ by —CH2 unit i.e., 14 mass units.
(ii) Each homologous series belongs to a similar class of compounds that show the same properties.
(b) Next higher homologue of C3H7OH is C4H9OH i.e., butanol.
Q.6. (a) Define the term isomers?
(b) Draw two possible isomers of the compound with molecular formula C3H6O and write their names.
(c) Give the electron dot structures of the above two compounds.
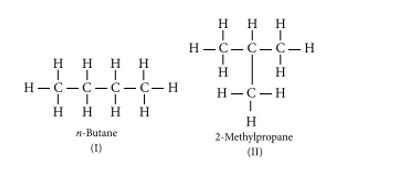
Ans. (a) Isomers are those molecules that have the same molecular formula but different structural formulas i.e., show different properties. For example, The structures of possible isomers of butane (C4H10) are:
The first three members of the alkane series are (i) CH4 (methane), (ii) C2H6 (ethane), and (iii) C3H8 (propane). In the above members of the alkane series, it is not possible to have different arrangements of carbon atoms. Thus, do not have isomers of first three members of alkane series.
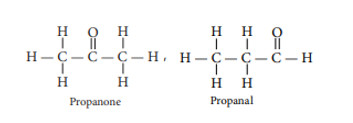
(b) Two possible isomers of the compound, C3H6O are:
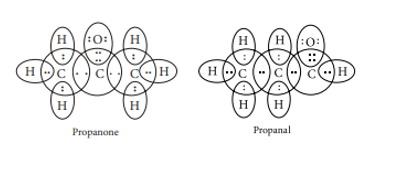
(c) The electron dot structures of propanone and propanal are:
As a result, the answers to the preceding six questions will help you ace your preparation and achieve the best results. All students taking the CBSE Term 2 exams should arm themselves with all of the necessary study resources, such as MTG’s 100 Percent Exam Ready CBSE Board Term 2 Chapter-wise Question Bank with detailed solutions. The questions in this book are both objective and subjective, and it also assures 99.99 percent concept clarity, which will eventually lead you to a perfect score.
Also Check – How to Prepare for CBSE Class 12 Term 2 Exams
Frequently asked questions
- How many chapters do I need to study for CBSE term 2 exam for class 10th science?
Ans.Total 7- Chapters;
Chapter – 4 Carbon and its compounds
Chapter – 5 Periodic Classification of elements
Chapter – 8 How do organisms reproduce?
Chapter – 9 Heredity and Evolution
Chapter – 12 Electricity
Chapter – 13 Magnetic effects of current
Chapter – 15 Our Environment - What is the time duration of the CBES Term 2 exam?
Ans. As per the CBSE notification released in July 2021, Term 2 will be of 2 hours. - Where I can find important questions of other chapters?
Ans. Must use MTG’s 100 Percent Exam Ready CBSE Board Term 2 Chapter-wise Question Bank with detailed solutions. In these books, you will find objective and subjective questions of the Term 2 chapters.