Many important questions are formed in competitive exams like CUET, JEE, NEET, and boards from Class 12 Physics. Below, we have provided Important Questions from Class 12 Physics Unit 8, Atoms and Nuclei for your JEE Mains 2023 preparation. You can check it here for revision purposes.
Latest: Dual Nature of Radiation and Matter Class 12 JEE Questions and Answers
Important: JEE Mains Previous Year Question Papers – For Exam 2023 | Free PDF Download
Also Check out: 30 Days JEE Main Planner to Boost Your JEE Main 2023 Preparation
Practice from – JEE Main Sample Papers 2023 With Answers: FREE PDF Download
Atoms and Nuclei Class 12 JEE Mains Questions and Answers
We’ve compiled a list of important questions about the Atoms and Nuclei Class 12 physics unit 8 that you should not miss while studying for your JEE Main Exams.
Q.1. A hydrogen-like atom (atomic number Z) is in a higher excited state of quantum number n. This excited atom can make a transition to the first excited state by successively emitting two photons of energies 10.20 eV and 17.00 eV respectively. Alternatively, the atom from the same excited state can make a transition to the second excited state by successively emitting two photons of energies 4.25 eV and 5.95 eV respectively. Determine the value of n. (Ionisation energy of hydrogen atom = 13.6 eV)
(a) n = 6 (b) n = 4 (c) n = 7 (d) n = 3
A.1. (a)
Q.2. Protons and singly ionized atoms of U235 and U238 are passed in turn (which means one after the other and not at the same time) through a velocity selector and then enter a uniform magnetic field. The protons describe semicircles of radius 10 mm. The separation between the ions of U235 and U238 after describing semicircle is given by
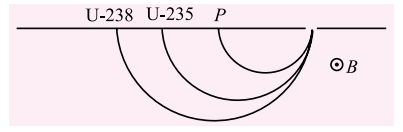
(a) 60 mm (b) 30 mm
(c) 2350 mm (d) 2380 mm
A.2. (a)
Q.3. Which of the following products in a hydrogen atom are independent of the principal quantum number n? The symbols have their usual meanings.
(a) vn (b) Er (c) En (d) vr
A.3. (a,b)
Q.4. Hydrogen atom in its ground state is excited by means of monochromatic radiation of wavelength 970.6 Å. How many different wavelengths are possible in the resulting emission spectrum?
A.4. (6)
Q.5. Starting with a sample of pure 66Cu, 7/8 of it decays into Zn in 15 minutes. Find the corresponding half-life (in minutes).
A.5. (5)
Q.6. An ∝-particle and a proton are accelerated from rest by a potential difference of 100 V. After this, their de Broglie wavelengths are λa and λp respectively. Find the ratio λp / λ∝, to the nearest integer.
A.6. (3)
Comprehension Type
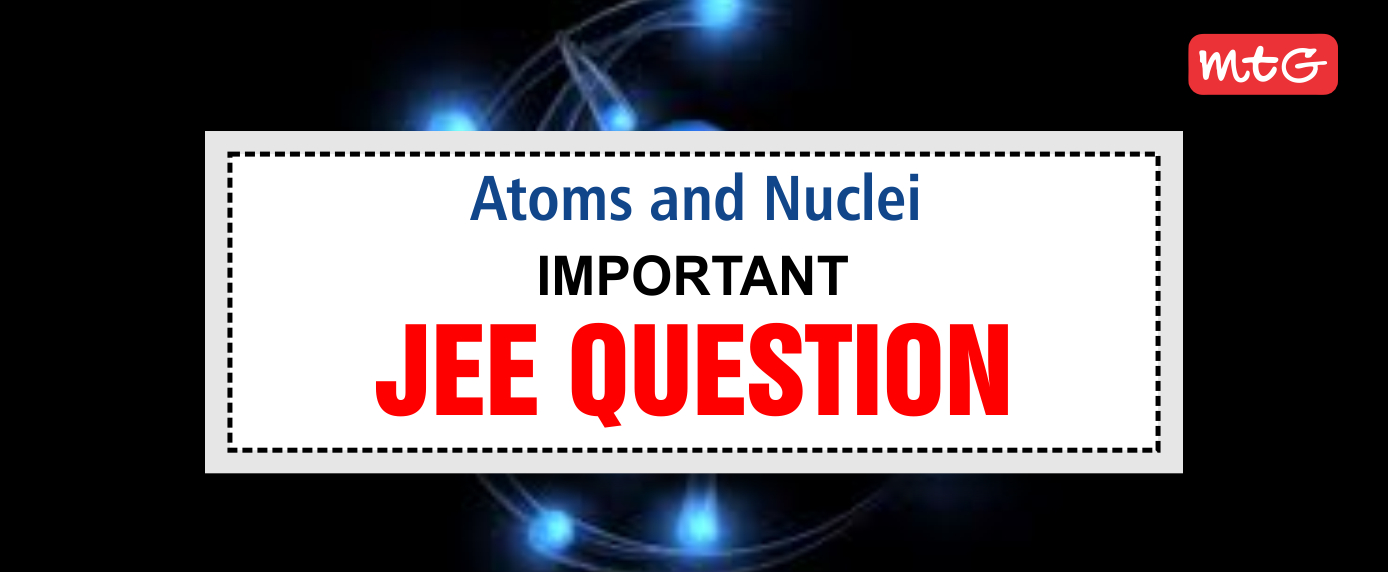
The key feature of Bohr’s theory of the spectrum of hydrogen atom is the quantization of angular momentum when an electron is revolving around a proton. We will extend this to a general rotational motion to find the quantized rotational energy of a diatomic molecule assuming it to be rigid. The rule to be applied is Bohr’s quantization condition.
A.1. (d)
A.2. (b)
Best JEE Books Recommended by JEE Toppers
Check out some of the best JEE Books recommended by JEE Toppers
- NCERT Textbook
One thing that is strongly recommended is that students prepare using NCERT textbooks. Toppers endorse NCERT as the finest book for JEE Main. NCERT is the foundation for these exams since they cover everything and consist of enormous information. Each and every line of NCERT must be clear and embedded in your mind to know the concepts better.
|
22 Years JEE Main Imagine having 21 years’ worth of experience for JEE Main? That is what you’ll get with this series of Physics, chemistry, and mathematics books. MTG’s 22 Years JEE Main Chapter-wise Topic-wise Solutions Physics is the best question bank having questions from the past 22 years of JEE Main Physics (2022-2013) & AIEEE (2012-2002) which are segregated Chapter-wise Topic-wise. |
|
JEE Champion JEE Champion is a book designed for JEE aspirants to prepare better with the knowledge of topic importance within a chapter. It familiarizes all the JEE aspirants with a wide variety of questions frequently asked in various engineering entrance exams. It covers 10 years of questions for all the major engineering exams. Students can understand the changing pattern of questions asked and can gain mastery over questions asked during exams.
|
|
46+22 Years Chapterwise Topicwise Solutions • 46 Previous Years’ Papers of JEE Advanced/IIT JEE |
All the Best!