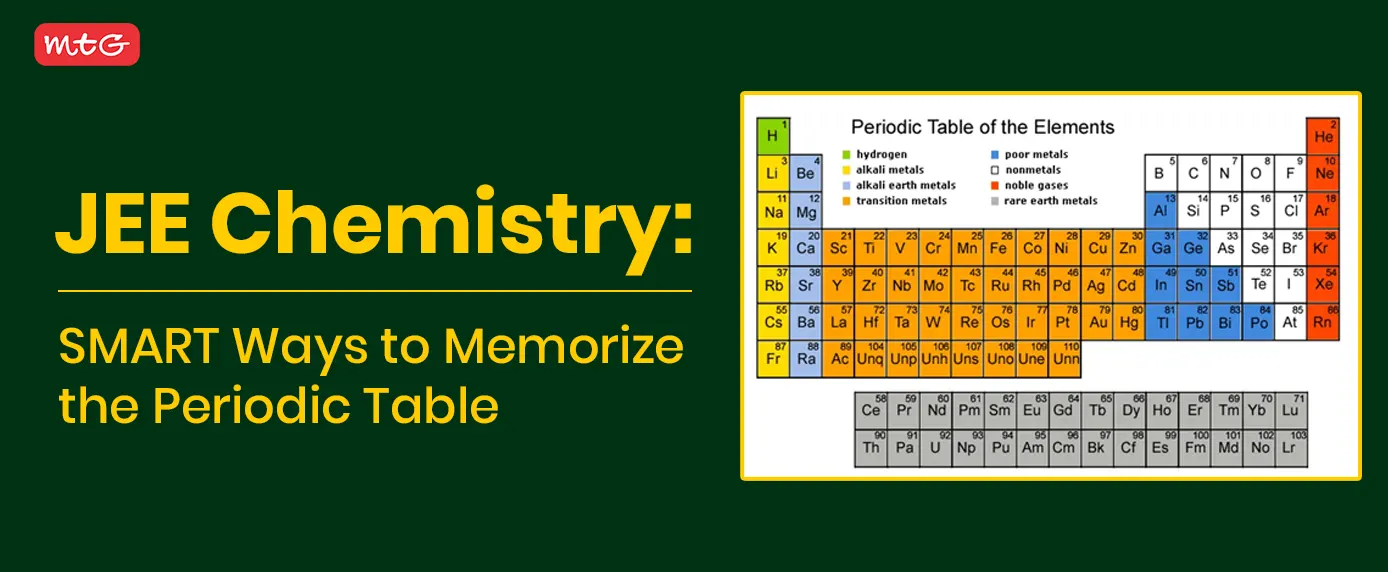
1. Why is memorizing the periodic table one of the first steps in mastering inorganic chemistry for JEE?
Memorizing the periodic table lays the foundation for understanding element behavior, trends, and relationships. Since questions often test your ability to predict reactivity, oxidation states, and compound formation, having the table at your fingertips saves time during exams and prevents errors. Inorganic chemistry concepts—like acid-base strength, redox reactions, and coordination chemistry—all hinge on knowing where an element sits in the table.
2. What’s the difference between natural and synthetic elements, and why does it matter for JEE prep?
-
Natural elements (Z=1–92): Found in nature (e.g., Hydrogen through Uranium). Most JEE questions focus on these because they’re more stable and better-studied.
-
Synthetic elements (Z=93–118): Man-made, often radioactive, with very short half-lives. While less common in standard JEE problems, knowing their names and general properties (radioactivity, rarity) can help in elimination-style questions.
3. How does the Flashcard Rotation method with spaced repetition work for learning all 118 elements?
-
Create flashcards listing each element’s name, symbol, atomic number, and key properties (oxidation states, common compounds, typical uses).
-
Review schedule:
-
Day 1: All cards
-
Day 4: Cards you struggled with
-
Day 7: Full deck again
-
Day 14, Day 30, etc.
-
-
Active recall: When you see the front (e.g., “Fe”), force yourself to name its properties before flipping. Over time, the increasing intervals move data into long-term memory.
4. What’s the most effective way to design flashcards for element properties (atomic number, symbol, oxidation states)?
-
Front side: Element name or symbol (e.g., “Iron” or “Fe”).
-
Back side:
-
Atomic number (e.g., 26)
-
Common oxidation states (e.g., +2, +3)
-
Key compounds (e.g., Fe₂O₃ for rust)
-
Typical uses (e.g., steel production)
-
Any special notes (e.g., “forms colored complexes in solution”)
By keeping each card focused, you test one core association at a time, which optimizes active recall.
-
5. How can colour-coding the periodic table improve my visual recall of element groups and trends?
-
Assign colours by category:
-
Reds for metals (Groups 1–2, and the d-block)
-
Blues for non-metals (Halogens, Noble Gases)
-
Greens for metalloids (B, Si, Ge, etc.)
-
-
Display prominently: Pin a large, colour-coded chart above your study desk.
-
Pattern reinforcement: The brain quickly associates “red” with metals and their shared traits (e.g., conductivity, malleability), making group identification almost instantaneous.
6. Which mnemonic techniques work best for remembering the order of elements in each group?
-
Create simple sentences where each word’s initial matches the element symbol.
-
Examples:
-
Group 1 (Alkali Metals): “Happy Little Naughty Kids Rarely Cry” → H, Li, Na, K, Rb, Cs, Fr
-
Group 17 (Halogens): “Fun Clowns Bring Intelligent Attentive Teachers” → F, Cl, Br, I, At, Ts
-
-
Practice aloud and write them once daily until they stick.
7. What are the key periodic trends (e.g., electronegativity, atomic radius) I need to focus on, and how can I remember them?
-
Electronegativity: Increases left → right across a period; decreases top → bottom down a group.
-
Atomic radius: Decreases left → right; increases top → bottom.
-
Ionization energy & electron affinity: Mirror electronegativity trends.
-
Memory aids:
-
“F–F–F” for Forward (→) Fall (radius)
-
“Down we grow” for radius increasing down a group
-
Sketch mini-diagrams of the table each week and annotate arrows for each trend.
-
8. How do I apply the Story-World Method to turn element groups into memorable narratives?
-
Pick a theme: E.g., Group 1 as superheroes.
-
Assign personalities:
-
Hydrogen: the lone hero, unpredictable
-
Lithium: the calm strategist
-
Sodium: the hot-headed brawler
-
-
Build a plot: Show how they team up (compound formation) or clash (reactivity with water).
-
Visualise key scenes: Draw or imagine comic panels of Cs (the most powerful) unleashing a giant water-splitting blast.
By embedding facts in a story, you tap into emotional memory, which is far more durable than rote data.
9. Are there quick-reference PDF downloads or resources you’d recommend for periodic table revision?
-
d-block overview PDF (Groups 3–12): Summarizes oxidation states and common coordination geometries.
-
Coordination compounds notes PDF: Key ligands, geometries, and color-change reactions.
-
JEE Main previous-year question PDFs: Use these to see how elements and trends are tested in real questions.
10. How should I integrate these memorization techniques with past JEE Main papers to reinforce active recall?
-
Morning session: 15 minutes of flashcard review.
-
Midday practice: Tackle a set of past-year questions focused on inorganic chemistry (e.g., 5–10 questions).
-
Evening wrap-up: Colour-coded table review or a 5-minute Story-World recap.
-
Weekly mock: Simulate a timed 20-question inorganic section, then analyze mistakes and update flashcards or mnemonics accordingly.
This blend of active recall, testing, and spaced repetition ensures you not only memorize facts but also apply them under exam conditions.