
To assist students in their preparation and help them perform better at JEE Mains Chemistry, MTG has come up JEE Mains Chemistry questions for the upcoming exam. These questions are likely to appear in JEE Mains, the ones considered most important. Questions are provided with answers. We will make sure to update every month. Check them out.
JEE Mains Important Chemistry Questions & Solutions – (19-07-2023)
Q.1. 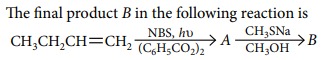
(a) 3-methylsulphanylbut-1-ene
(b) allyl methyl sulphide
(c) n-butyl methyl sulphide
(d) diallyl sulphide.
A.1. (a)
Q.2. Which of the following expressions correctly represents the relationship between the average molecular kinetic energies (KE ) of CO and N2 molecules at the same temperature?
(a) KECO = KEN2 (b) KECO > KEN2
(c) KECO < KEN2 (d) All of the above
A.2. (a)
Q.3. The BCl3 is a planar molecule whereas NCl3 is pyramidal because
(a) N—Cl bond is more covalent than B—Cl bond
(b) B—Cl bond is more polar than N—Cl bond
(c) nitrogen atom is smaller than boron
(d) BCl3 has no lone pair but NCl3 has a lone pair of electron.
A.3. (d)
Q.4. The standard reduction potentials of Cu2+/Cu and Cu2+/Cu+ are 0.337 V and 0.153 V respectively. The standard electrode potential of Cu+ /Cu half cell is
(a) 0.184 V (b) 0.827 V
(c) 0.521 V (d) 0.490 V
A.4. (c)
Q.5. Which of the following orders of relative strengths of acids is correct?
(a) FCH2COOH > ClCH2COOH > BrCH2COOH
(b) ClCH2COOH > BrCH2COOH > FCH2COOH
(c) BrCH2COOH > ClCH2COOH > FCH2COOH
(d) ClCH2CO2H > FCH2COOH > BrCH2COOH
A.5. (a)
Q.6. The standard heat of combustion of Al is –837.8 kJ mol–1 at 25 °C. If Al reacts with O2 at 25°C, which of the following will release 250 kJ of heat?
(a) The reaction of 0.624 mole of Al.
(b) The formation of 0.624 mole of Al2O3.
(c) The reaction of 0.313 mol of Al.
(d) The formation of 0.150 mol of Al2O3.
A.6. (d)
Q.7. Which of the following statements is not correct?
(a) La(OH)3 is less basic than Lu(OH)3.
(b) In lanthanide series ionic radius of Ln3+ ions decreases.
(c) La is actually an element of transition series rather than lanthanide series.
(d) Atomic radii of Zr and Hf are same because of lanthanide contraction.
A.7. (a)
JEE Mains Important Chemistry Questions & Solutions – (16-06-2023)
Q.1. How many moles of ferric alum (NH4)2SO4⋅Fe2(SO4)3.24H2O can be made from the sample of Fe containing 0.0056 g of it?
(a) 10–4 mol (b) 0.5 × 10–4 mol
(c) 0.33 × 10–4 mol (d) 2 × 10–4 mol
A.1. (b)
Q.2. aK2Cr2O7 + bKCl + cH2SO4 xCrO2Cl2 + yKHSO4 + zH2O
The above equation balances when
(a) a = 2, b = 4, c = 6 and x = 2, y = 6, z = 3
(b) a = 4, b = 2, c = 6 and x = 6, y = 2, z = 3
(c) a = 6, b = 4, c = 2 and x = 6, y = 3, z = 2
(d) a = 1, b = 4, c = 6 and x = 2, y = 6, z = 3
A.2. (d)
Q.3. In the decomposition of N2O5, the plot between the reciprocal of concentration of the reactant and the time was found to be linear as shown in the figure. Determine the order of reaction.
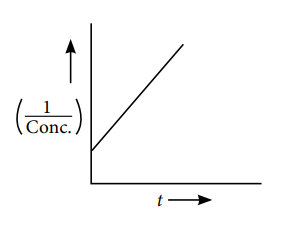
(a) First (b) Second
(c) Zero (d) None of these
A.3. (b)
Q.4. Which one among the following will show the tautomerism?
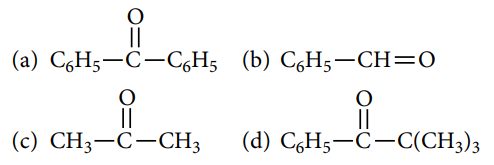
A.4. (c)
Q.5. The correct figure representing isothermal and adiabatic expansions of an ideal gas from a particular initial state is
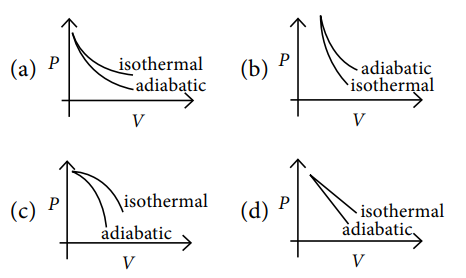
A.5. (a)
Q.6. The products expected to be formed in the Wurtz reaction of a mixture of neopentyl bromide and isobutyl bromide are
(i) 2,2,4-trimethylpentane
(ii) 2,2,5,5-tetramethylhexane
(iii)2,2,4,4-tetramethylhexane
(iv) 2,5-dimethylhexane
(v) 2,2,5-Trimethylhexane
(a) (ii), (iii) and (v) (b) (ii), (iv) and (v)
(c) (i), (iv) and (v) (d) (i), (iii) and (v)
A.6. (b)
Q.7. Identify the correct statement regarding enzymes.
(a) Enzymes are specific biological catalysts that can normally function at very high temperatures (T ~ 1000 K)
(b) Enzymes are normally heterogeneous catalyststhat are very specific in action.
(c) Enzymes are specific biological catalysts thatcannot be poisoned.
(d) Enzymes are specific biological catalysts thatpossess well-defined active sites.
A.7. (d)
Q.8. Which of the following options is incorrect?
(a) pH at half equivalent point of a weak acid (HA) is equal to pKb of its conjugate base.
(b) An aqueous solution of sodium acetate will be basic due to anionic hydrolysis.
(c) On dilution of a solution of a weak acid, degree of ionisation will increase but [H+] will decrease.
(d) All of these.
A.8. (a)
JEE Mains Some Basic Concepts of Chemistry|Structure of Atom Chaptwewise Physics Questions with Solutions – (24-5-2023)
Check out JEE Main important Chapterwise Chemistry Questions on Some Basic Concepts of Chemistry|Structure of Atom for the month of February.
Q.1. Which element has a hydrogen-like spectrum whose lines have a wavelength one-fourth of atomic hydrogen?
(a) He+ (b) Li2+ (c) Be3+ (d) B4+
A.1. (a)
Q.2. If the threshold frequency of a metal for photoelectric effect is u0, then which of the following will not happen?
(a) If frequency of the incident radiation is u0, then kinetic energy of the electrons ejected is zero
(b) If frequency of incident radiation is u, then kinetic energy of the electrons ejected will be hu–hu0.
(c) If frequency is kept same at u, but intensity is increased, then number of electrons ejected will increase.
(d) If frequency of incident radiation is further increased, then number of electrons ejected will increase.
A.2. (d)
Q.3. Three isotopes of an element have mass numbers, M, (M + 1) and (M + 2). If the mean mass number is (M + 0.5) then which of the following ratio may be accepted for M, (M + 1), (M + 2) in that order
(a) 1: 1: 1 (b) 4: 1: 1 (c) 3: 2: 1 (d) 2: 1: 2
A.3. (b)
Q.4. With increasing principal quantum number, the energy difference between adjacent energy levels in H atom
(a) decreases (b) increases
(c) remains constant
(d) decreases for low value of Z and increases for higher value of Z
A.4. (a)
Q.5. Assertion: The value of n for a line in Balmer series of hydrogen spectrum having the highest wavelength is 4 and 6.
Reason: For Balmer series of hydrogen spectrum, the value n1 = 2 and n2 = 3, 4, 5.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
A.5. (d)
Q.6. The frequency of radiation emitted when the electron falls from n = 4 to n = 1 in a hydrogen atom will be (Given ionization energy of H = 2.18 × 10–18 J atom–1 and h = 6.625 × 10–34 J s)
(a) 1.54 × 1015 s–1
(b) 1.03 × 1015 s–1
(c) 3.08 × 1015 s–1
(d) 2.00 × 1015 s–1
A.6. (c)
Important JEE Main Questions
JEE Mains Chemistry Questions with Solutions
JEE Mains Physics Questions with Solutions
JEE Mains Maths Questions with Solutions
Q.7. 12 g of Mg (atomic mass = 24) on reacting completely with acid gives hydrogen gas, the volume of which at STP would be
(a) 22.4 L (b) 11.2 L (c) 44.8 L (d) 6.1 L
A.7. (b)
Q.8. The de-Broglie wavelength of a particle with mass 1 g and velocity 100 m/s is
(a) 6.6 × 10–33 m (b) 6.6 × 10–34 m
(c) 6.6 × 10–35 m (d) 6.6 × 10–35 m
A.8. (a)
Q.9. The maximum number of electrons in a subshell is given by the expression
(a) 4l – 2 (b) 4l + 2 (c) 2l + 1 (d) 4n2
A.9. (b)
Best JEE Books Recommended by JEE Toppers
- NCERT Textbook
One thing that is strongly recommended is that students prepare using NCERT textbooks. Toppers endorse NCERT as the finest book for JEE Main. NCERT is the foundation for these exams since they cover everything and consist of enormous information. Each and every line of NCERT must be clear and embedded in your mind to know the concepts better.
|
Imagine having 21 years’ worth of experience for JEE Main? That is what you’ll get with this series of Physics, chemistry, and mathematics books. MTG’s 22 Years JEE Main Chapter-wise Topic-wise Solutions Physics is the best question bank having questions from the past 22 years of JEE Main Physics (2022-2013) & AIEEE (2012-2002) which are segregated Chapter-wise Topic-wise. |
|
JEE Champion is a book designed for JEE aspirants to prepare better with the knowledge of topic importance within a chapter. It familiarizes all the JEE aspirants with a wide variety of questions frequently asked in various engineering entrance exams. It covers 10 years of questions for all the major engineering exams. Students can understand the changing pattern of questions asked and can gain mastery over questions asked during exams.
|
|
46+22 Years Chapterwise Topicwise Solutions • 46 Previous Years’ Papers of JEE Advanced/IIT JEE |
All the Best!



































